On April 30, 2021, the FDA published an announcement regarding a recall of multiple lots of Acella’s NP Thyroid product. Unlike some recent recalls of thyroid products, for example those manufactured by RLC Labs, this recall included information on adverse events reported to Acella. According to the release, Acella has received 43 reports of serious adverse events that could possibly be related to this recall at the time it was published. This recall follows a separate recall in 2020 of lots of NP Thyroid which were found to be super-potent.
What is NP Thyroid?
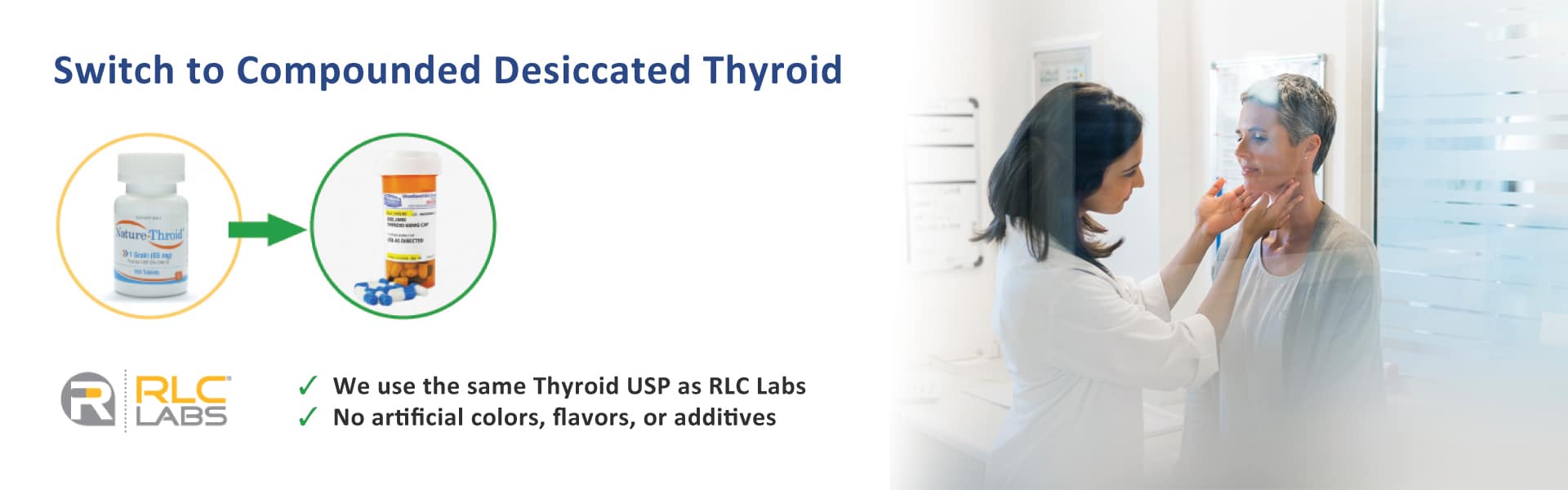
NP Thyroid is one brand-name version of Thyroid USP – also referred to as natural desiccated thyroid. Natural desiccated thyroid contains both T4 (levothyroxine) and T3 (liothyronine) as well as naturally occurring thyroid cofactors T1, T2, calcitonin and iodine (in trace amounts). The inactive ingredients are calcium stearate, dextrose (agglomerated) and mineral oil. Thyroid USP itself is the active ingredient in every commercially available natural desiccated thyroid medication. It is also the active ingredient in compounded NDT capsules. Natural desiccated thyroid is most often obtained from the thyroid glands of pigs so it tends to have some natural variability between batches.
Why Were NP Thyroid Lots Recalled?
Similar to the RLC Labs recall of Nature Throid and WP Thyroid, Acella recalled lots of NP Thyroid due to sub-potency. This is defined as a product containing less than 90% of the labeled amount of liothyronine (T3) and/or levothyroxine (T4). There are numerous risks for people with thyroid conditions who take a sub-potent dose of their medication. When a patient is given medication for hypothyroidism and the dose is less than is needed, they may experience a return of symptoms of hypothyroidism that were being treated. These side effects may include fatigue, increased sensitivity to cold, constipation, dry skin, puffy face, hair loss, slow heart rate, depression, swelling of the thyroid gland and/or unexplained weight gain or difficulty losing weight. In elderly patients and patients with underlying cardiac disease toxic cardiac manifestations of hyperthyroidism may occur, such as cardiac pain, palpitations or cardiac arrhythmia.
Acella has reported at least 43 patients who have had serious adverse events from taking the sub-potent doses of NP Thyroid. There may be more unreported cases, or cases that will be reported in the coming weeks. The voluntary, nationwide recall will hopefully prevent any more adverse events from occurring.
March 2020 NP Thyroid Recall
It is important to note that a separate recall was issued in 2020 for lots of NP Thyroid that were found to be super-potent (up to 115% of labeled amount of T3 and/or T4). This FDA announcement was released on May 22, 2020 – view the May 2020 announcement here (opens in new window).
This recall was not as large as the more recent recall. It included less than half as many lots and at the time of publication of the announcement only 2 adverse events had been reported. Both sub-potent and super-potent doses of natural desiccated thyroid can results in side effects.
What Lots Were Recalled?
Over 30 lots have been recalled which were distributed from around March 2020 until March 2021. These include lots of 15mg, 30mg, 60mg, 90mg, and 120mg tablets (these are all of the dosages currently manufactured). The full list of recalled lot numbers can be viewed in the FDA announcement: view April 30, 2021 announcement (opens in new window).
It is important for patients to know that they should not discontinue any thyroid medication until they consult with their healthcare provider. The side effects of discontinuing without finding an alternative to switch to may be more severe.
Why Natural Desiccated Thyroid?
Brand-name NDT products have experienced fairly consistent recalls in recent years. Because they are derived from natural sources, they tend to have variability in potency between batches which needs to be managed. So why do patients continue to take them? Most patients who are taking these medications have already tried other types of thyroid medication and they have not worked. For these patients, the combination of T4, T3, and thyroid co-factors helps them alleviate their hypothyroid symptoms better than synthetic versions of T4. In most cases the benefit compared to other medications has been so significant with NDT, that patients are willing to change brands repeatedly even through multiple recalls. In studies it has been shown that even when participants were blinded to whether they were taking synthetic thyroid or NDT, they preferred the NDT more often than they preferred synthetic.
Compounded Natural Desiccated Thyroid
An alternative to name-brand NDT medications is a compounded version that includes the same active ingredient – Thyroid USP. A compounding pharmacy can make NDT in any dosage required to match the dosage of a name-brand medication. A compounding pharmacy can make capsules in any dosage, which may help customize treatment to the individual in how they take their medication on a daily basis.
If You Were Affected by the Recall
If you have a lot number of NP Thyroid subject to the recall, you can email Acella Pharmaceuticals at recall@acellapharma.com or contact one of their representatives at 1-888-424-4341, Monday through Friday from 8:00 am – 5:00 pm ET. Consumers should contact their physician or healthcare provider if they have experienced any problems that may be related to taking or using this drug product.







WHEN IS NATURE THIROID BACK ON THE MARKET????? This is beyond RIDICULOUS!!! Those of us who PREFER NATURAL THYROID MEDS ARE BEING SHORT CHANGED AND THIS NEEDS TO STOP!
We have no estimated date from RLC Labs when Nature Throid will be in production again – or if it will be discontinued. Other natural thyroid brands have also experienced recalls. Our pharmacy is compounding NDT in capsules in any dosage required.
Joyce, I agree with you!
Do insurance companies pay for natural thyroid medications?
Insurance coverage for compounded medications will depend on your plan. We would suggest checking for coverage before you order. We can provide a claims form with your order if you would like to try to get your prescription reimbursed.
I just paid $ 122.76 for my NP Thyroid 120 mg. I took it back to the pharmacy when I received the recall notice.
How can I get a refund for the money I spent on this medicine? I have my receipt.
I can not afford to take this loss.
Thank you
Susan Comberger
Acella Pharmaceuticals is the manufacturer of NP Thyroid. You should reach out to them with any questions about the recall. There is more information on this page: https://npthyroid.com/product-updates/
This is 2 years of recalls on thyroid meds I can barely function!! I don’t understand why it’s so hard to make a thyroid pill that actually works!! Nature Thyroid was amazing I felt great! I complained to my doctor for a year (still paying) when I found out the company was sold a year ago!! I knew something was wrong but my doctors blew it off and still collected my money I had to research to find the truth! Now this thyroid pill has been recalled for 3 months!!!
Our pharmacy does compound an alternative to Nature Throid and can make it in the same dosage you were using before.